Abstract
Introduction: Idelalisib (IDELA) is a selective PI3Kδ inhibitor approved for use with rituximab in patients with previously treated chronic lymphocytic leukemia (CLL). Reversible transaminase elevation (TAE) has been observed in all CLL clinical trials involving IDELA, with frequencies of Gr ≥3 elevation in company sponsored Ph 2 and 3 studies utilizing the approved dose of 150 mg orally twice a day, ranging broadly from approximately 10% (Sharman ASH 2014) to 41% (Hillmen EHA 2017). TAE leads to permanent discontinuation of IDELA in approximately 3% of patients (Coutre EHA 2015). Risk factors for Gr ≥3 TAE are not well understood. Lampson et al. (Blood 2016) reported a trend toward increased risk in younger patients and those with mutated IGHV in a series of 24 previously untreated CLL patients who received IDELA monotherapy. In the present study, we analyze the rates of Gr ≥3 TAE across all fully enrolled, Gilead sponsored Ph 2 and 3 studies with the goal of identifying risk factors related to patient and treatment characteristics and selected CLL genetic alterations.
Methods: Data were reviewed from patients who received at least 1 dose of IDELA in any of 6 Ph 2 or 3 studies, 3 for treatment naïve (TN) and 3 for relapsed/refractory (R/R) CLL. The following variables were used for analysis: age, prior therapy (Y vs N), combination with bendamustine plus rituximab (BR; Y vs N), del(17p) (Y vs N), and IGHV mutated vs unmutated.
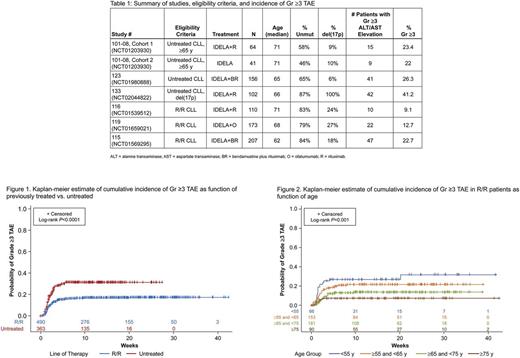
Results: A total of 853 patients were enrolled and treated with IDELA (Table 1) and comprise the safety analysis set (SAS). The median age was 67 y (range: 37-90), and the median IDELA exposure time was 11 mo (range: <1-43). Of those patients experiencing Gr ≥3 TAE, the median time to onset was 8 weeks. TN patients were at greater risk than R/R (Figure 1; HR=2; log rank p<0.0001). The other risk factors were then evaluated separately in the TN and R/R groups, as well as in the entire SAS. Significantly increased risk was found in younger patients enrolled in R/R trials (Figure 2; log rank p=0.001) and also within the entire SAS (log rank p=0.013). For patients in R/R trials, treatment with BR was a significant risk factor (log rank p=0.0005); although patients receiving BR were younger, the increased risk was seen across the age ranges. In the TN population, del(17p) was a risk factor (log rank p=0.02), but this relationship is mainly driven by the 17p-specific Study 133 and thus may be confounded by a study-related effect. All other analyses yielded p-values greater than 0.05. The incidence of Gr ≥3 TAE in the placebo groups of the R/R studies was 2.2% overall, 3.8% for BR, and 1.1% for non-BR-containing regimens.
Conclusions: In this ad hoc analysis of 853 patients treated in Gilead sponsored trials of IDELA, younger age and TN status were risk factors for developing Gr ≥3 elevation of hepatic transaminases, as was treatment with BR for patients with R/R CLL. The highest incidence of Gr ≥3 TAE was observed in a single-arm trial of previously untreated patients with del(17p). IGHV mutation status was not a significant risk factor in this dataset. A statistical model of risk factors for Gr ≥3 TAE will be presented, considering interactions between the factors.
Brown: Infinity Pharmaceuticals: Consultancy; Pharmacyclics: Consultancy; Sun BioPharma: Consultancy, Research Funding; Astellas Pharma: Consultancy; Redx: Consultancy; Janssen: Consultancy; Celgene: Consultancy; AbbVie: Consultancy, Honoraria; Roche/Genentech: Consultancy; Janssen Oncology: Honoraria; Gilead: Consultancy, Research Funding; Pfizer: Consultancy; AstraZeneca: Consultancy. Zelenetz: Genentech Roche: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Mei Pharma: Research Funding; Hospira: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda Pharma: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Adaptive Biotech: Consultancy, Honoraria. Furman: TG Therapeutics: Consultancy; Verstem: Consultancy; Gilead Sciences: Consultancy; Abbvie: Consultancy, Honoraria; Sunesis: Consultancy; Pharmacyclics: Consultancy, Speakers Bureau; Genentech: Consultancy. Lamanna: Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding. Mato: DTRM: Research Funding; Pharmacyclics: Research Funding; Portola: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy; AstraZeneca: Consultancy; Janssen: Consultancy; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Research Funding; Regeneron: Research Funding; AbbVie: Consultancy, Research Funding. Montillo: Janssen: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; Gilead Sciences, Inc.: Consultancy, Honoraria, Speakers Bureau; Roche: Research Funding; Novartis: Honoraria; Genetech: Research Funding. O'Brien: CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Alexion: Consultancy; Acerta: Other: Research Support: Honorarium, Research Funding; GSK: Consultancy; TG Therapeutics: Consultancy, Other: Research Support: Honorarium, Research Funding; Pharmacyclics: Consultancy, Other: Research Support: Honorarium, Research Funding; Pfizer: Consultancy, Research Funding; Janssen: Consultancy; Vaniam Group LLC: Consultancy; AbbVie: Consultancy; ProNAI: Other: Research Support: Honorarium, Research Funding; Celgene: Consultancy; Regeneron: Other: Research Support: Honorarium, Research Funding; Gilead Sciences, Inc.: Consultancy, Other: Research Support: Honorarium, Research Funding; Sunesis: Consultancy; Astellas: Consultancy; Aptose Biosciences, Inc.: Consultancy. Dreiling: Gilead Sciences, Inc.: Employment. Dubowy: Gilead Sciences, Inc.: Employment, Equity Ownership. Kim: Gilead Sciences, Inc.: Employment. Munugalavada: Gilead Sciences, Inc.: Employment, Equity Ownership. Robak: Janssen: Consultancy, Honoraria, Research Funding; Akari Therapeutics Plc: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Hillmen: Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Celgene: Research Funding; Roche: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal